
Hi, help us enhance your experience
Hi, help us enhance your experience
Hi, help us enhance your experience
965 Views
eMediNexus 25 October 2022
Increased neurohumoral activation in heart failure induces a state of increased sodium and water acidity, resulting in an increased plasma volume. Novel therapies that induce diuresis and increase the success of decongesting AHF with volume overload are needed. The pathophysiological evidence suggests that acetazolamide enhances the efficiency of loop diuretics in this regard.
The Acetazolamide in Decompensated heart failure with Volume OveRload (ADVOR) trial is a multicentre, randomized, double-blind, placebo-controlled study designed to investigate if acetazolamide combined with loop diuretic therapy improves decongestion in AHF with volume overload.
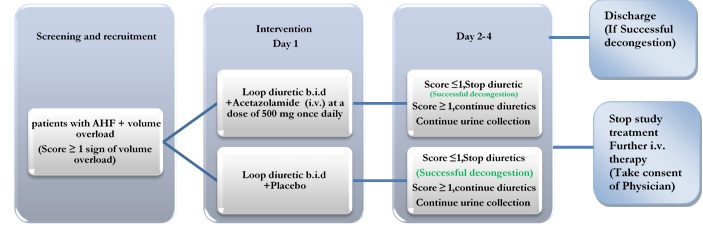
The primary endpoint is successful decongestion with no more than trace edema assessed on the third morning of admission, with good diuretic efficacy (urine output >3.5 L during the first 30–48 h of decongestive treatment).
Secondary endpoints included all-cause mortality or heart failure readmission after three months, length of hospital stay for the index admission, and longitudinal changes in the EuroQol-5 questionnaire.
If the ADVOR trial is successful, off-patent medicine could revolutionize the treatment of congestion and volume overload worldwide. In addition, it will prepare the way for a future trial with more definitive results in AHF.
Reference: Mullens W, Verbrugge FH, Nijst P, et al. Rationale and design of the ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) trial. Eur J Heart Fail. 2018;20(11):1591-1600.
{{Article_Title}}
{{Article_Author}}
{{Article_Title}}
{{Article_Author}}